Scroll to:
Development of a Method for Obtaining Nanoscale Magnesium Carbonate Stabilized with Chitosan as the Basis of Scaffold Matrices for Regenerative Medicine
https://doi.org/10.23947/2687-1653-2024-24-4-392-401
EDN: KXALAI
Abstract
Introduction. In the public domain there is enough literature on methods of treating the musculoskeletal system. The possibilities of eliminating bone defects using patients' own (autologous) bones are described. The authors of theoretical and applied studies also suggest using synthetic bioinert materials made of polymers, calcium phosphates, plastics, and metals. The creation of three-dimensional matrices based on scaffolds for the formation of systems that are as close as possible to bone tissue in structure has been studied. It is known that the active substances of the scaffold matrix can be hydroxyapatite, tricalcium phosphate, as well as silicates, carbonates of magnesium, calcium, copper, zinc, and manganese. The issue requires detailed study. In light of the stated problem, the features of the listed materials should be considered separately. There are no such publications. The presented work is intended to fill this gap. Its objective is to create a synthesis method and study the properties of nanoscale magnesium carbonate.
Materials and Methods. The materials for the study were samples of magnesium carbonate nanoparticles obtained by chemical precipitation in water. They were studied using X-ray diffractometry, scanning electron microscopy, infrared spectroscopy, and dynamic light scattering. Quantum-chemical modeling was performed using the QChem program and the IQmol molecular editor.
Results. It has been established that magnesium carbonate particles are rod-shaped, 2 to 10 μm in length. They consist of nanoparticles from 30 to 60 nm. Quantum-chemical modeling has revealed the energy features of the interaction of the basic magnesium carbonate, firstly, with chitosan with carbonate, and secondly, with a separate chitosan molecule. In the first case, the energy value is lower, in the second, it is higher. This indicates the chemical and energetic advantage of forming such complexes. The corresponding indices for the optimal coordination of magnesium carbonate with chitosan have been determined. In this case, the interaction is provided by the hydroxyl group of chitosan attached to the C6 residue of glucosamine. For this process, the lowest energy ∆E=462.387 kcal/mol and chemical hardness η=0.062 eV are noted. Magnesium carbonate nanoparticles have optimal radius and zeta potential with the following parameters of the initial reagents: 0.018 mol of ammonium carbonate, 0.03 mol of magnesium acetate, 0.15 g of chitosan.
Discussion and Conclusion. The obtained data indicate that nanoscale basic magnesium carbonate is a promising material with a wide range of possibilities of practical application. From this point of view, its role in metabolic processes, namely in the assimilation of macronutrients, is of particular interest. Nanoscale osteotropic magnesium micronutrient synthesized in a biopolymer environment can be used as a biologically active filler for three-dimensional scaffold matrices. Implementation of this solution in medical practice will improve the efficiency of bone tissue restoration.
Keywords
For citations:
Blinov A.V., Rekhman Z.A., Gvozdenko A.A., Yasnaya M.A., Kolodkin M.A., Taravanov M.A. Development of a Method for Obtaining Nanoscale Magnesium Carbonate Stabilized with Chitosan as the Basis of Scaffold Matrices for Regenerative Medicine. Advanced Engineering Research (Rostov-on-Don). 2024;24(4):392-401. https://doi.org/10.23947/2687-1653-2024-24-4-392-401. EDN: KXALAI
Introduction. Regenerative medicine constantly requires materials that help accelerate osteogenesis [1]. Even with the emergence of new solutions, the problem remains urgent, since fractures are a very common type of injury [2]. It should be noted that patients fully recover in only 16% of cases. According to the World Health Organization, there are about 50 million severe injuries worldwide that cause the loss of labor capacity and invalidity. Therefore, the treatment of bone tissue defects is a pressing medical and social problem [3]. To help the patient, three-dimensional biopolymer matrices based on scaffolds are used. They contain elements that are close in structure to connective bone tissue. One of such materials for regenerative medicine is nanoscale forms of magnesium carbonate [4]. Magnesium is an essential microelement [5]. It is responsible for the strength of bones [6] and participates in their formation [7]. Orthopedic implants are created on its basis [8]. In nanoscale form, magnesium carbonate has the following properties:
- low toxicity;
- good biocompatibility;
- permeability for drugs [9].
In biological interactions, the roughness and chemical composition of the surface of the elements play an important role [10]. Future materials based on compounds in the nanometer range may eventually change the nature of the tissues around the implant and increase the clinical success of this approach [11]. Biopolymers are used to improve the above properties. One of them is chitosan [12]. It is an important biocompatible component of connective tissue. It dissolves and decomposes well [13].
The main objective of this study is to create a method for synthesizing chitosan-stabilized nanoscale magnesium carbonate and to study its properties. The material is considered as the basis for scaffold matrices for regenerative medicine.
Materials and Methods. Using chemical precipitation, magnesium carbonate nanoparticles were synthesized from a magnesium-containing precursor, magnesium acetate. The precipitant was ammonium carbonate, and the stabilizer was the polysaccharide chitosan. At the first stage, the required volume of 1% chitosan solution was added to the magnesium acetate solution. Then, using a dropping funnel, the precipitant solution was introduced into the precursor solution at a rate of 60 drops per minute with constant stirring. After the entire precipitant solution had been added, the resulting sol was stirred for another 10 minutes. The synthesized sol was centrifuged and then dried in a drying chamber. This is how magnesium carbonate powder samples were obtained. Their phase composition was studied using powder diffractometry on an Empyrean device (PANalytical, the Netherlands) with the following measurement parameters:
- copper cathode (radiation wavelength — 1.54 Å);
- measurement range — 10–90° 2θ;
- sampling rate — 0.01°2θ.
The microstructure of magnesium carbonate samples was studied using a MIRA3-LMH scanning electron microscope (Tescan, Czech Republic).
To prepare the samples, carbon double-sided conductive tape was placed on the instrument table (12 mm), powder of the material under study and a carbon layer 10 nm thick were applied. Measurement parameters:
- accelerating voltage — 10 kV;
- focal length value — 4.9 mm;
- In-Beam SE detector.
For the computer quantum chemical modeling of magnesium carbonate in the interaction with chitosan, QChem software was used. The Hartree-Fock method and the basis set 6–31G1 were selected for the research. The molecular editor IQmol [14] was used to configure the molecules. The samples were examined by infrared (IR) spectroscopy. For this purpose, an IR spectrometer with Fourier transform was used, FSM 1201 model (Russia).
Powders MgCO3 and KBr were thoroughly mixed in a ratio of 1:300 and pressed into a tablet in a special press mold under a pressure of 500–1000 MPa. The resulting samples were placed in a spectrometer, and measurements were taken within the range of 400–4400 cm–1.
The average hydrodynamic radius of chitosan-stabilized magnesium carbonate nanoparticles was studied using the dynamic light scattering method on a Photocor complex device (Russia). Their ζ-potential was estimated by the acoustic and electroacoustic spectroscopy method on a DT–1202 spectrometer (Dispersion Technology Inc., USA).
The method for obtaining magnesium carbonate nanoparticles was optimized through a multifactorial experiment. The following parameters were used:
- three variables (content of magnesium acetate, ammonium carbonate, and chitosan);
- two outputs (average hydrodynamic radius and zeta potential) [15].
The data were processed using the Statistica 10.0 program. The levels of variation are presented in Table 1.
Table 1
Levels of Variation of Variables
|
Name of parameters, designations |
Levels of variation of variables |
||
|
Magnesium acetate content, mol |
0.012 |
0.024 |
0.030 |
|
Ammonium carbonate content, mol |
0.012 |
0.024 |
0.030 |
|
Mass of chitosan, g |
0.150 |
0.300 |
0.450 |
Next, an experimental planning matrix was constructed (Table 2).
Table 2
Experimental Planning Matrix
|
No |
Volume of 0.8 M acetate solution магния, мл |
Volume of 0.8 M carbonate solution аммония, мл |
Volume of chitosan solution, ml |
|
1 |
15 |
15 |
15 |
|
2 |
15 |
30 |
30 |
|
3 |
15 |
45 |
45 |
|
4 |
30 |
15 |
30 |
|
5 |
30 |
30 |
45 |
|
6 |
30 |
45 |
15 |
|
7 |
45 |
15 |
45 |
|
8 |
45 |
30 |
15 |
|
9 |
45 |
45 |
30 |
Research Results. At the first stage, the phase composition of the obtained samples was studied. The results are presented in Figure 1.
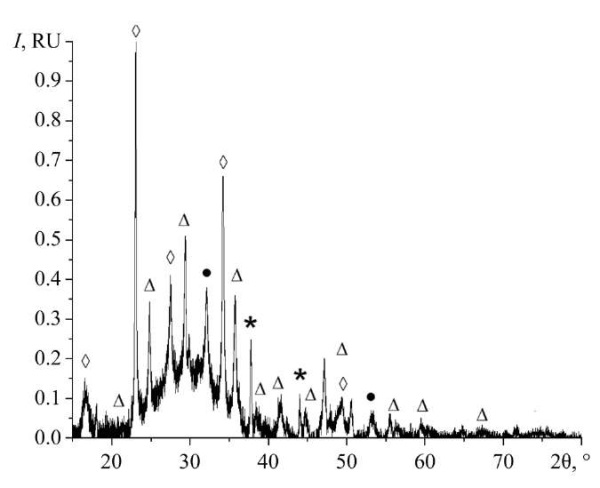
Fig. 1. Diffraction pattern of a sample of nanoscale magnesium carbonate stabilized with chitosan. Here, ● — MgCO3, ◊ — Mg2(CO3)(OH)2 ·3H2O, ∆ — MgCO3 · 5H2O,* — MgO
Next, the microstructure of the obtained samples of magnesium carbonate nanoparticles stabilized with chitosan was examined using a scanning electron microscope (SEM) (Fig. 2).
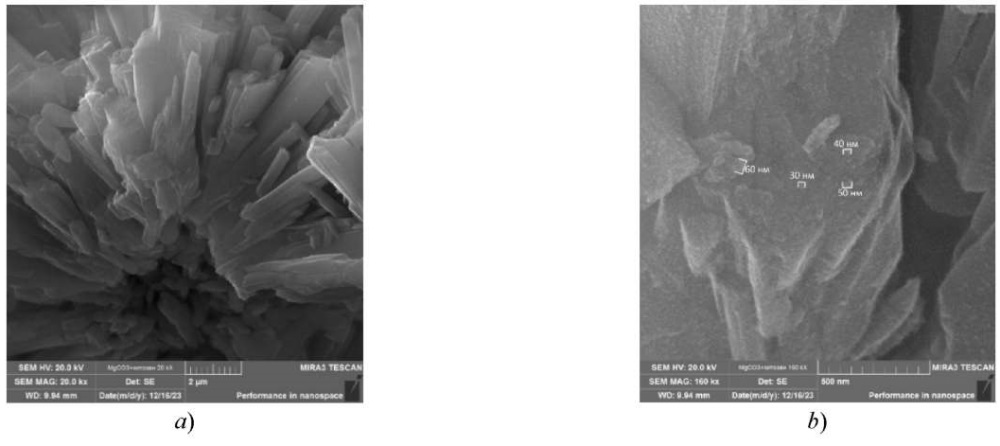
Fig. 2. SEM micrographs of a sample of magnesium carbonate nanoparticles stabilized with chitosan: a — magnification 20,000 times; b — magnification 160,000 times
To study the method of coordination of magnesium carbonate nanoparticles with chitosan, the quantum chemical modeling of the molecular structures of magnesium carbonate with chitosan was performed. The results are shown in Table 3 and Figures 3, 4. Here, HOMO is the highest occupied molecular orbit, LUMO is the lowest unoccupied molecular orbital.
Table 3
Results of Quantum Chemical Calculations of Molecular Structures of Magnesium Carbonate and Basic Magnesium Carbonate with Chitosan
|
Interaction |
Type of magnesium compound |
E, kcal/mol |
∆E, kcal/mol |
EHOMO, eV |
ELUMO, eV |
η, eV |
|
Monomeric unit of chitosan |
– |
–1258.049 |
– |
–0.225 |
0.030 |
0.128 |
|
Through hydroxyl group that is attached to C6 residue of glucosamine |
MgCO3 |
–1720.436 |
462.387 |
–0.161 |
–0.037 |
0.062 |
|
Mg(OH)2CO3 |
–1994.103 |
736.054 |
–0.179 |
–0.111 |
0.034 |
|
|
Through hydroxyl group that is attached to C3 residue of glucosamine |
MgCO3 |
–1720.366 |
462.317 |
–0.167 |
–0.042 |
0.063 |
|
Mg(OH)2CO3 |
–1994.273 |
736.224 |
–0.182 |
–0.064 |
0.059 |
|
|
Through amino group which is attached to C2 residue of glucosamine |
MgCO3 |
–1720.418 |
462.369 |
–0.124 |
–0.019 |
0.053 |
|
Mg(OH)2CO3 |
–1994.104 |
736.055 |
–0.156 |
–0.048 |
0.054 |
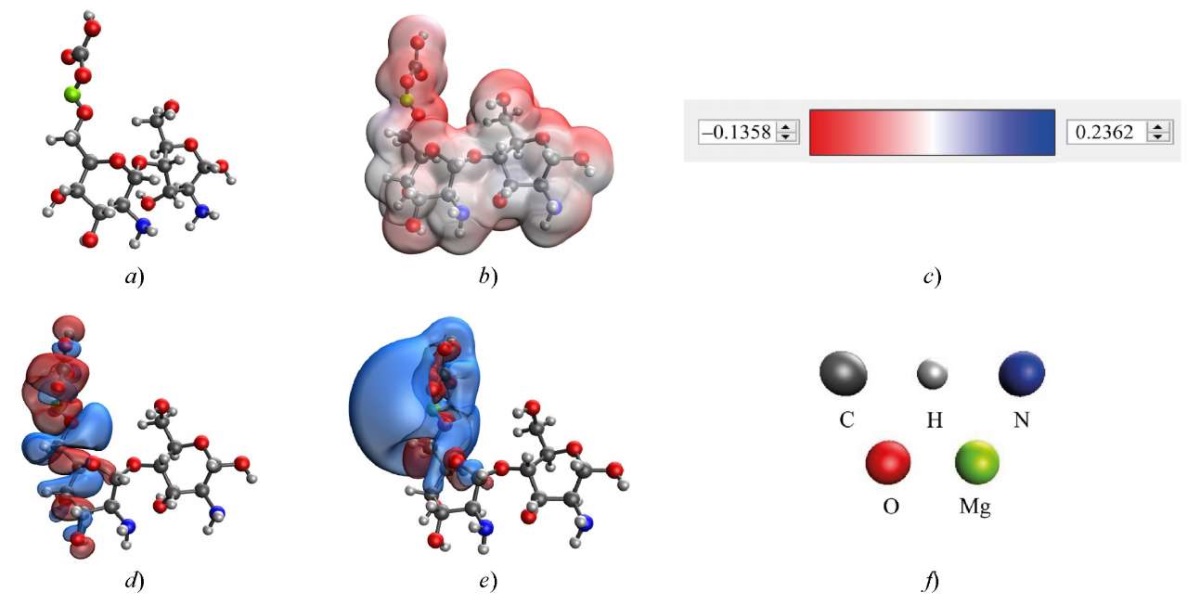
Fig. 3. Results of modeling the interaction of chitosan with magnesium carbonate through hydroxyl group attached to C6 residue of glucosamine in chitosan: a — model of the complex; b — electron density distribution; c — gradient of electron density distribution; d— HOMO; e — LUMO; f — decoding of atoms
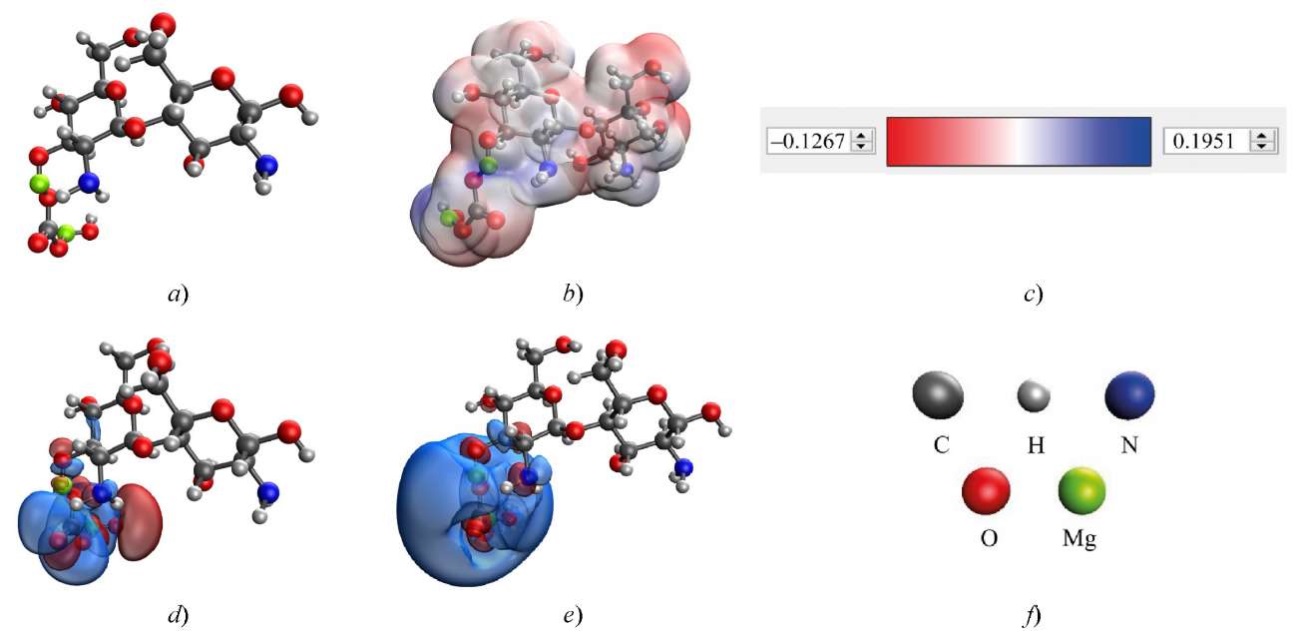
Fig. 4. Results of modeling the interaction of chitosan with basic magnesium carbonate through hydroxyl group attached to C3 residue of glucosamine in chitosan: a — model of the complex; b — electron density distribution; c — gradient of electron density distribution; d— HOMO; e — LUMO; f — decoding of atoms
To validate the quantum chemical modeling data, the samples were examined using infrared spectroscopy. The results are shown in Figure 5.
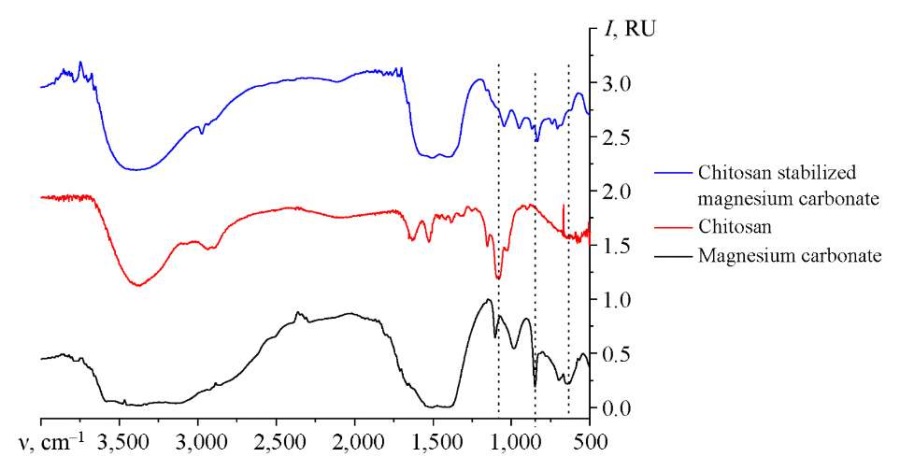
Fig. 5. IR spectrum of magnesium carbonate nanoparticles stabilized with chitosan
To study the influence of input parameters on the synthesis of nanoscale magnesium carbonate, ternary dependences were formed. Figure 6 shows the dependence of the average hydrodynamic radius of nanoparticles on the content of the initial reagents.
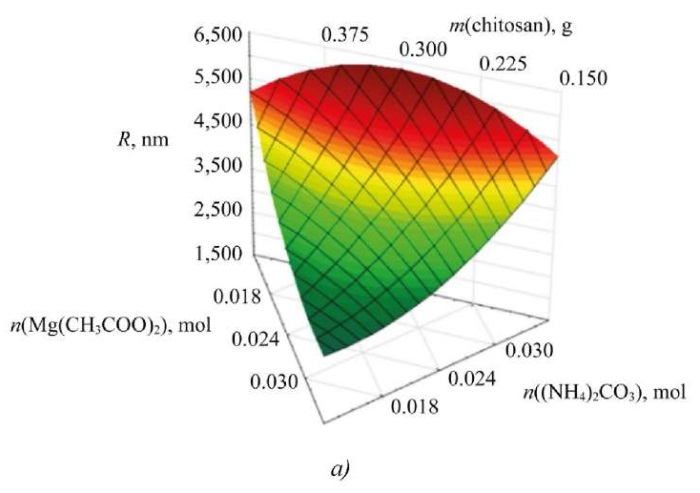
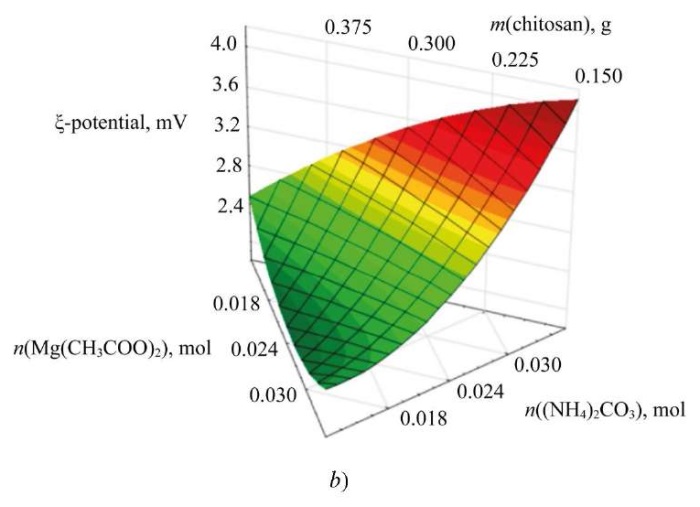
Fig. 6. Dependences of the change in average hydrodynamic radius and electrokinetic potential on concentrations of initial reagents: a — ternary surface describing the effect of initial reagents on particle size of magnesium carbonate; b — ternary surface describing the effect of initial reagents on electrokinetic potential of magnesium carbonate particles
Analysis of the phase composition of the samples has shown that there are phases of anhydrous magnesium carbonate (MgCO3), two configurations of magnesium carbonate in the form of crystal hydrate (MgCO3 · 5H2O), Mg2(CO3)(OH)2·3H2O and magnesium oxide (MgO).
When analyzing the surface microstructure of magnesium carbonate nanoparticles, it is found that the sample has an anisotropic shape. Magnesium carbonate is represented by rod-shaped particles from 2 to 10 μm in length, which consist of nanoparticles from 30 to 60 nm in size.
The computer quantum chemical modeling allowed us to compare the energy of interaction with the basic magnesium carbonate:
- chitosan with carbonate;
- a separate chitosan molecule.
In the first case, the energy value was lower than in the second. This indicates the chemical and energetic benefit of forming such complexes (for magnesium carbonate, the interaction energy is more than 462.00 kcal/mol, and for the basic carbonate — more than 736.00 kcal/mol).
In the optimal variant of coordination of magnesium carbonate with chitosan, the interaction occurred through the hydroxyl group of chitosan attached to C6 residue of glucosamine. This interaction had the lowest energy ∆E = 462.387 kcal/mol and chemical hardness η = 0.062 eV.
The position of the functional groups was determined by IR spectroscopy of chitosan-stabilized magnesium carbonate nanoparticles. Additionally, the spectra of pure chitosan and magnesium carbonate were recorded. The analysis of the IR spectrum of magnesium carbonate showed that in the region from 2200 to 3000 cm–1 there were the stretch vibrations of the groups NH3+, NH2+, NH+ and CH2, –CH3. The bands at 988 cm–1, 1102 cm–1, 1414 cm–1 and 1529 cm–1 were associated with vibrations of C–O and C=O in group CO32 [16]. The band at 620 cm–1 corresponded to vibrations of the hydroxyl group, and the bands at 698 and 852 cm–1 were specified by vibrations of the bond Mg–O [17].
The analysis of the IR spectra of chitosan showed that the region from 2500 to 3400 cm–1 was responsible for the stretching vibrations of the following functional groups: –OH, –CH3, CH2 [18]. The region from 1000 to 1900 cm–1 characterized the vibrations of bonds C–O, C–O–C, –CH2, –CH3, C–N, NH2+ [19]. The region of bands from 500 to 900 cm–1 referred to deformation vibrations: at 898 cm–1 – bonds C–H [20], at 581, 652, 704 and 768 cm–1 — bonds –CH and –CH2.
As the analysis of the sample of magnesium carbonate nanoparticles stabilized with chitosan has shown, the range from 2100 to 3000 cm–1 contains stretching vibrations of the groups NH3+, NH2+, NH+, –CH3, CH2, O–H. This is typical for the chitosan molecule. There are also bands at 1414 cm–1 and 1529 cm–1, which correspond to vibrations C–O and C=O in group CO32, which confirms the presence of magnesium carbonate functional groups in the system [21]. The binding of magnesium is confirmed by the presence of deformation vibrations C–O and C=O in group CO32– and bond vibrations Mg–O and CO32– in the range from 700 to 1100 cm–1 [22].
A decrease in the intensity of the peaks at 620 cm–1 and 1078 cm–1, which correspond to the vibrations of groups O–H and C–O, is noted. This indicates the interaction of magnesium carbonate and chitosan through hydroxyl groups and is consistent with the results of quantum chemical modeling.
The study on the ternary surfaces obtained suggests that the change in the ratio between magnesium acetate and ammonium carbonate affects significantly the size and zeta potential of magnesium carbonate particles [23]. The average hydrodynamic radius of the particles does not depend on the chitosan content. However, the change in zeta potential is related to the stabilizer content and precipitant concentration, which is important for assessing the stability of nanoscale systems. As a result, the parameters of the initial reagents are selected, at which the sample of magnesium carbonate nanoparticles has an optimal radius and zeta potential:
- 018 mol ammonium carbonate;
- 0.03 mol magnesium acetate;
- 0.15 g chitosan.
Discussion and Conclusion. Magnesium in magnesium carbonate is needed for the normal functioning of the body. In metabolic processes, it maintains the effective absorption of macronutrients. This allows us to talk about the urgency and potential demand for the method of obtaining nanoscale magnesium carbonate stabilized with chitosan. This approach is developed and optimized within the framework of the presented research. It has been established that magnesium carbonate nanoparticles are rod-shaped agglomerates with a length of 2 to 10 μm. These clusters consist of nanoparticles with a size of 30 to 60 nm. The optimal way of coordinating molecules is the interaction of magnesium carbonate through the hydroxyl group in C6 residue of glucosamine in the chitosan molecule. The advantage is provided by significant indicators of energy and chemical rigidity.
IR spectroscopy of samples of magnesium carbonate nanoparticles stabilized with chitosan revealed a decrease in the intensity of the bands that characterize the vibrations of group O–H (for magnesium carbonate) and the vibrations of group C–O (for chitosan). It follows from this that the interaction occurs through the hydroxyl groups of chitosan.
The optimization of the method for synthesizing magnesium carbonate nanoparticles stabilized with chitosan, performed within the framework of the presented research, allows us to make a number of statements.
- With increasing magnesium acetate content, the particle size and electrokinetic potential of nanoscale magnesium carbonate decrease.
- With the growth of concentration of ammonium carbonate, the average hydrodynamic radius and surface charge of magnesium carbonate nanoparticles increase.
- The concentration of chitosan has a slight effect on the radius of the particles, but as its content increases, the surface charge decreases.
1. Here, 6–31G is the basis set used in this research. STO-nG is a family of Slater-type orbital basis sets.
References
1. Kanev AA, Kurakov FA, Cherchenko OV, Tsvetkova LA. The Development of Regenerative Medicine in Russia and in the World: Leading Researchers and Technological Drivers. Economics of Science. 2022;8(3–4):202–219. https://doi.org/10.22394/2410–132X–2022–8–3–4–202–219
2. Safronova TV. Inorganic Materials for Regenerative Medicine. Inorganic Materials. 2021;57(5):443–474. https://doi.org/10.1134/S002016852105006X
3. Agazade AR, Agazade RR, Gergieva TF, Amkhadov IS, Kadiev AA, Mamedov SE, et al. Evaluation of the Effectiveness of Treatment and Monitoring of Patients with Systemic Disorders of Bone Tissue during Dental Implantation. Medical Alphabet. 2023;1(1):44–49. https://doi.org/10.33667/2078–5631–2023–1–44–49
4. Golubeva AN. Proper Nutrition as the Main Component of a Healthy Lifestyle. International Journal of Humanities and Natural Sciences. 2023;76(1–4):40–42. https://doi.org/10.24412/2500–1000–2023–1–4–40–42
5. Pogozheva AV, Kodentsova VM, Sharafetdinov KhKh. The Role of Magnesium and Potassium in Preventive and Therapeutic Nutrition. Problems of Nutrition. 2022;91(5):29–42. https://doi.org/10.33029/0042–8833–2022–91–5–29–42
6. Kochneva EV. Magnesium Deficiency in Clinical Practice. Nutrition. 2018;8(1):37–51. https://doi.org/10.20953/2224–5448–2018–1–37–51
7. Evseeva GP, Suprun SV, Suprun EN, Rakitskaya EV, Kozlov VK, Lebed’ko OA. Influence of Trace ElementsImbalance on Immunity. Trace Elements in Medicine. 2021;22(S1):27–28. https://doi.org/10.19112/2413–6174–2021-S1–12
8. Hang Zhou, Bing Liang, Haitao Jiang, Zhongliang Deng, Kexiao Yu. Magnesium-Based Biomaterials as Emerging Agents for Bone Repair and Regeneration: From Mechanism to Application. Journal of Magnesium and Alloys. 2021;9(3): 779–804. URL: https://www.jmamg.com/uploadfiles/2024/04/20240407095328708.pdf (accessed: 25.06.2024).
9. Rubnikovich SP, Khomich IS. The Use of Bone Grafts and Bone Substitutes to Eliminate Defects and Augment Jaw Bones in Dental Implantology and Periodontology. Dentist. 2014;1(12):77–86. URL: http://journal-stomatolog.by/wpcontent/uploads/2018/05/2–13–2014.pdf (accessed: 25.06.2024).
10. Monich SG, Khramkova AS, Bondarenko VA. The Use of Nanotechnology in Dental Implantology. In: Proc. 16th International Science and Technology Conference “Instrumentation-2023”. Minsk: BNTU Publ.; 2023. P. 288–289. (In Russ.) https://rep.bntu.by/handle/data/138532
11. Volova LT, Trunin DA, Ponomareva YuV, Popov NV. Study of Biocompatibility and Cytotoxicity of Personalized Bone Implants Using Cell Technologies. Bulletin of REAVIZ: Rehabilitation, Doctor, and Health. 2017;29(5):32–39.
12. Kou Sh(G), Peters L, Mucalo M. Chitosan: A Review of Molecular Structure, Bioactivities and Interactions with the Human Body and Micro-Organisms. Carbohydrate Polymers. 2022;282:119132. https://doi.org/10.1016/j.carbpol.2022.119132
13. Wenjie Wang, Changhu Xue, Xiangzhao Mao. Chitosan: Structural Modification, Biological Activity and Application. International Journal of Biological Macromolecules. 2020;164:4532–4546. https://doi.org/10.1016/j.ijbiomac.2020.09.042
14. Blinov AV, Pirogov MA, Gvozdenko AA, Golik AB, Rekhman ZA, Kolodkin MA. Computer Quantum-Chemical Modeling of the Interaction of Selenium Nanoparticles with Quaternary Ammonium Compounds. Physical and Chemical Aspects of the Study of Clusters, Nanostructures and Nanomaterials. 2023;15:357–366. https://doi.org/10.26456/pcascnn/2023.15.357
15. Anisimov AV. Experiment Planning as an Effective Method of Technological Process Optimization. In: Proc. International Science and Technology Conference “Current Issues in Veterinary Medicine, Food and Biotechnology”. Saratov: Saratov State Vavilov Agrarian University Publ.; 2022. P. 249–252. (In Russ.)
16. Frost RL. Raman Spectroscopic Study of the Magnesium Carbonate Mineral Hydromagnesite (Mg5[(CO3)4 OH)2]·4H2O). Journal of Raman Spectroscopy. 2011;42(8):1690–1694. https://doi.org/10.1002/jrs.2917
17. Kornprobst T, Plank J. Synthesis and Properties of Magnesium Carbonate Xerogels and Aerogels. Journal of Non-Crystalline Solids. 2013;361:100–105. https://doi.org/10.1016/j.jnoncrysol.2012.10.023
18. Aksay S. Effects of Al Dopant on XRD, FT-IR and UV–vis Properties of MgO Films. Physica B: Condensed Matter. 2019;570:280–284. https://doi.org/10.1016/j.physb.2019.06.020
19. Apfelbaum F, Mayer I, Rey C, Lebugle A. Magnesium in Maturing Synthetic Apatite: A Fourier Transform Infrared Analysis. Journal of Crystal Growth. 1994;144(3-4):304–310. https://doi.org/10.1016/0022–0248(94)90471–5
20. Frost RL, Reddy BJ, Bahfenne S, Graham J. Mid-Infrared and Near-Infrared Spectroscopic Study of Selected Magnesium Carbonate Minerals Containing Ferric Iron – Implications for the Geosequestration of Greenhouse Gases. Spectrochimica Acta. Part A: Molecular and Biomolecular Spectroscopy. 2009;72(3):597–604. https://doi.org/10.1016/j.saa.2008.10.043
21. Zawadzki J, Kaczmarek H. Thermal Treatment of Chitosan in Various Conditions. Carbohydrate Polymers. 2010;80(2):394–400. https://doi.org/10.1016/j.carbpol.2009.11.037
22. Silva SML, Braga CRC, Fook MVL, Raposo CMO, Carvalho LH, Canedo EL. Application of infrared spectroscopy to analysis of chitosan/clay nanocomposites. In book: Th Theophanides (ed). Infrared Spectroscopy — Materials Science, Engineering and Technology. Ch. 3. London: IntechOpen Publ.; 2012. P. 43–62. http://doi.org/10.13140/2.1.3806.5609
About the Authors
A. V. BlinovRussian Federation
Andrey V. Blinov, Cand.Sci. (Eng.), Head of the Department of Physics and Technology of Nanostructures and Materials
1, Pushkin Str., Stavropol, 355017
Z. A. Rekhman
Russian Federation
Zafar A. Rekhman, Teaching Assistant of the Department of Physics and Technology of Nanostructures and Materials
1, Pushkin Str., Stavropol, 355017
A. A. Gvozdenko
Russian Federation
Alexey A. Gvozdenko, Teaching Assistant of the Department of Physics and Technology of Nanostructures and Materials
1, Pushkin Str., Stavropol, 355017
M. A. Yasnaya
Russian Federation
Maria A. Yasnaya, Cand.Sci. (Chemistry), Associate Professor, the Department of Physics and Technology of Nanostructures and Materials
1, Pushkin Str., Stavropol, 355017
M. A. Kolodkin
Russian Federation
Maxim A. Kolodkin, Head of the Laboratory Complex of the Department of Physics and Technology of Nanostructures and Materials
1, Pushkin Str., Stavropol, 355017
M. A. Taravanov
Russian Federation
Maxim A. Taravanov, Assistant of the Department of Physics and Technology of Nanostructures and Materials
1, Pushkin Str., Stavropol, 355017
Review
For citations:
Blinov A.V., Rekhman Z.A., Gvozdenko A.A., Yasnaya M.A., Kolodkin M.A., Taravanov M.A. Development of a Method for Obtaining Nanoscale Magnesium Carbonate Stabilized with Chitosan as the Basis of Scaffold Matrices for Regenerative Medicine. Advanced Engineering Research (Rostov-on-Don). 2024;24(4):392-401. https://doi.org/10.23947/2687-1653-2024-24-4-392-401. EDN: KXALAI
JATS XML














































